Intrinsic Semiconductors Explained with Properties and Examples
Think of a material so clean it has no impurities. This is what is intrinsic semiconductor. It is a pure material without added elements or chemicals. It’s interesting because it is the base of modern electronics.
Intrinsic semiconductors are important in technology. They naturally control how electricity flows. They have equal amounts of electrons and holes. These help them carry electricity in certain conditions. This makes them key to understanding devices like diodes and transistors.
Key Takeaways
Intrinsic semiconductors are pure materials without any added impurities.
They have the same number of electrons and holes to control electricity.
Their conductivity changes with temperature. Cold makes them insulators.
Heat lets electricity move through them more easily.
Silicon and germanium are examples of intrinsic semiconductors with special traits.
These semiconductors are important for devices like diodes and transistors.
Scientists use them to study and improve semiconductor technology.
Knowing about intrinsic semiconductors helps make better electronics and clean energy.
Picking a semiconductor depends on its bandgap, heat resistance, and purpose.
What Is an Intrinsic Semiconductor?
Definition of Intrinsic Semiconductor
An intrinsic semiconductor is a pure material with no impurities. It is made of one element or compound, like silicon or germanium. Its structure is balanced, meaning electrons and holes are equal. Holes are empty spaces left when electrons move.
The ability to conduct electricity depends on temperature. At 0 Kelvin, these semiconductors act as insulators because electrons can't move. When the temperature increases, heat gives electrons energy to move. They jump from the valence band to the conduction band, allowing electricity to flow.
Property/Definition | Description |
|---|---|
Heat gives electrons energy to move and conduct electricity. | |
Behavior at Low Temperature | At 0 Kelvin, they act as insulators. |
Electron-Hole Balance | Equal numbers of electrons and holes keep the material neutral. |
Fermi Level | Shows the chance of electrons being in energy bands. |
Characteristics of Intrinsic Semiconductors
Intrinsic semiconductors have special traits that make them useful in electronics:
Chemical Purity: They have no impurities, so they work consistently.
Equal Charge Carriers: Electrons and holes are always in equal amounts.
Temperature Sensitivity: Conductivity rises with heat due to thermal excitation.
Material Composition: Examples include silicon, germanium, and gallium arsenide.
These features make intrinsic semiconductors great for studying how semiconductors work. They are also used in research to create advanced electronics.
How Intrinsic Semiconductors Function
How do intrinsic semiconductors work? Their function depends on moving electrons and holes. When the temperature rises, electrons gain energy and move to the conduction band. This leaves holes in the valence band, keeping electrons and holes balanced.
The intrinsic carrier concentration (ni) measures the number of electrons and holes. This depends on the material and temperature. For instance, silicon has a lower ni than germanium, making it more stable in heat.
Intrinsic semiconductors are vital in devices like diodes and transistors. These devices need precise control of electrons and holes to work well. The purity and balance of intrinsic semiconductors ensure they perform reliably. This is why they are used in electronics and cars.
Did You Know?
Products like memory chips and circuits often use intrinsic semiconductors because they are reliable.
Properties of Intrinsic Semiconductors
Band Structure of Intrinsic Semiconductors
The band structure is key to how intrinsic semiconductors work. These materials have two energy bands: the valence band and the conduction band. The valence band holds electrons stuck to atoms. The conduction band has free electrons that move and carry electricity. Between these bands is the bandgap, where no electrons are found.
In intrinsic semiconductors, the bandgap is small. This lets electrons jump from the valence band to the conduction band when they get enough energy, like from heat. For example, silicon's bandgap is about 1.1 eV, while germanium's is smaller at 0.66 eV. These small gaps make them great for electronics.
Property | Description |
|---|---|
Band Structure | A small bandgap allows electrons to move between bands. |
Conductivity | Some electrons jump to the conduction band at room temperature. |
Temperature Effects | Heat changes the number of electrons and holes, affecting conductivity. |
Scientists have studied band structures more deeply. For example, a model for 3C-SiC includes orbital effects and spin-orbit coupling. This model matches experiments and helps explain band diagrams better.
Charge Carriers: Electrons and Holes
Intrinsic semiconductors depend on two charge carriers: electrons and holes. Electrons are negatively charged, while holes are positively charged spaces left when electrons move. These carriers are always equal in number, keeping the material neutral.
When electrons gain energy, they move to the conduction band. This leaves holes in the valence band. The movement of electrons and holes creates an electric current. Measuring these carriers can be tricky. For example, Hall effect measurements may not always give accurate results.
Hall mobility might seem lower than the real carrier mobility.
Hall carrier density can sometimes appear higher than the actual carrier count.
Learning about these carriers helps you understand how semiconductors work in devices like diodes and transistors.
Temperature Effects on Intrinsic Semiconductor Conductivity
Temperature greatly affects how intrinsic semiconductors conduct electricity. At 0 Kelvin, they act as insulators because no electrons can move. As the temperature rises, heat gives electrons energy to jump the bandgap. This lets them carry electricity.
This temperature-based behavior makes intrinsic semiconductors very useful. For example, silicon's carrier concentration grows quickly with heat. This is why silicon stays reliable in many electronic uses.
Feature | Description |
|---|---|
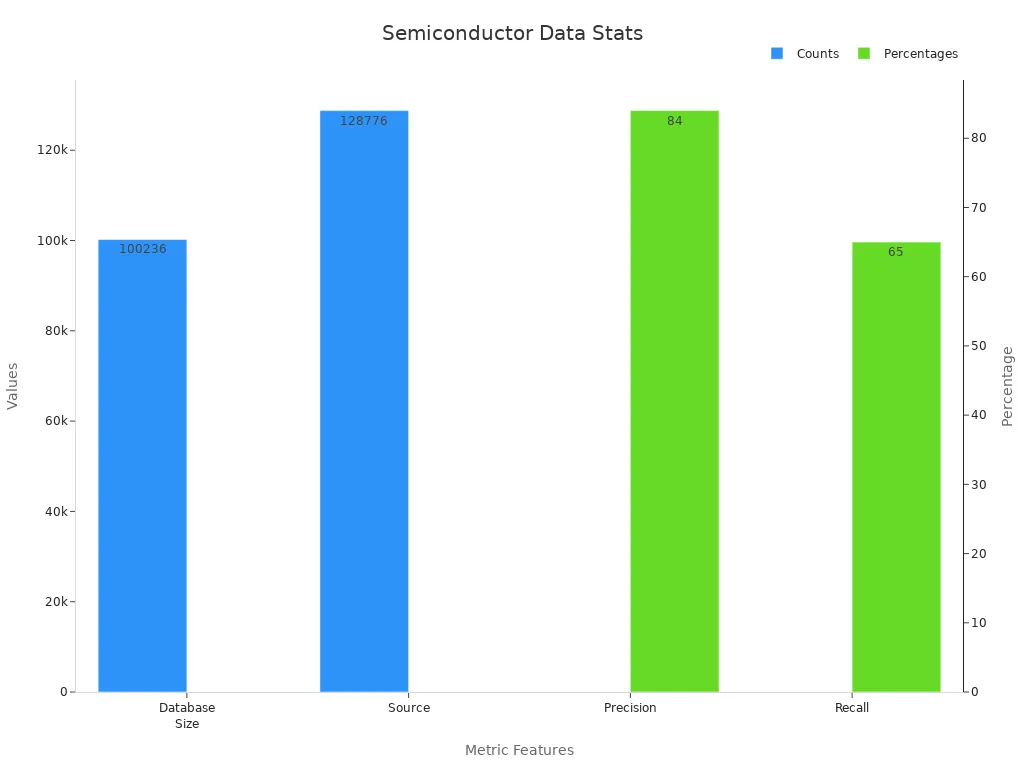
Database Size | 100,236 semiconductor band gap records |
Source | Curated from 128,776 journal articles |
Methodology | Uses ChemDataExtractor with machine learning |
Precision | 84% |
Recall | 65% |
Formats Available | CSV, JSON, MongoDB |
By understanding how temperature affects them, you can see why intrinsic semiconductors are so important. Their ability to adjust to different conditions makes them essential in modern technology.
Electrical Conductivity in Intrinsic Semiconductors
Electrical conductivity in intrinsic semiconductors shows how pure materials carry electricity. Unlike metals, which use free electrons, these semiconductors use both electrons and holes. This balance makes them special and important for modern devices.
How Electrical Conductivity Works
In intrinsic semiconductors, electrons and holes move to create conductivity. Electrons gain energy and jump to the conduction band. This leaves holes behind, which act as positive charges. Together, they form an electric current.
Temperature affects how well these semiconductors conduct electricity. At low temperatures, electrons can't move, so the material acts like an insulator. As heat increases, electrons get excited and conductivity improves.
Tip:
Higher temperatures mean more electrons and holes to carry charge. This makes intrinsic semiconductors sensitive to heat.
Factors Affecting Conductivity
Several things impact the conductivity of intrinsic semiconductors:
Material Type: Silicon and germanium are common examples. Silicon has a bigger bandgap, making it less conductive than germanium at room temperature.
Temperature: Heat gives electrons energy to move, boosting conductivity.
Carrier Concentration: More electrons and holes improve how well the material conducts electricity.
Factor | Impact on Conductivity |
|---|---|
Material Type | Affects bandgap size and ease of electron movement. |
Temperature | Higher heat increases carrier concentration and conductivity. |
Carrier Concentration | More carriers lead to better electrical conductivity. |
Why Electrical Conductivity Matters
Learning about conductivity in intrinsic semiconductors shows their importance in devices like diodes and transistors. These parts need careful control of charge carriers to work properly. The steady behavior of intrinsic semiconductors makes them great for electronics research.
By understanding conductivity, you can see how intrinsic semiconductors help technology. Their ability to handle temperature changes and keep electrons and holes balanced ensures reliable use in many applications.
Examples of Intrinsic Semiconductors
Silicon as an Intrinsic Semiconductor
Silicon is a very common intrinsic semiconductor. It is found in most modern electronics. Its atomic structure helps it conduct electricity in certain conditions. Silicon has four valence electrons. These electrons form strong bonds with nearby atoms in its crystal structure.
At absolute zero, silicon acts like an insulator. Its electrons stay tightly bound. When the temperature increases, heat excites the electrons. This energy allows them to move to the conduction band. This creates free electrons and holes, enabling electricity to flow.
Silicon’s bandgap is about 1.1 eV, making it stable at room temperature. This stability is why silicon is used in transistors, solar cells, and circuits. It is also abundant and affordable, making it popular in the semiconductor industry.
Fun Fact: Silicon is the second most common element in the Earth's crust. This makes it an eco-friendly choice for electronics.
Germanium as an Intrinsic Semiconductor
Germanium is another example of an intrinsic semiconductor. It is similar to silicon but has unique traits. Germanium’s bandgap is smaller, around 0.66 eV. This means it needs less energy for electrons to move to the conduction band.
Because of its smaller bandgap, germanium conducts electricity better at lower temperatures. However, it is less stable in high heat. Germanium was important in the early days of semiconductors. It was used in the first transistors and diodes before silicon became more popular.
Germanium has high carrier mobility, making it useful for certain tasks. It is used in high-speed electronics and infrared optics. Its ability to detect infrared light makes it valuable for sensors and optical devices.
Other Examples of Intrinsic Semiconductors
Besides silicon and germanium, other materials are intrinsic semiconductors. Gallium arsenide (GaAs) is one example. It has a direct bandgap, which helps it absorb and emit light well. This makes it great for devices like LEDs and laser diodes.
Diamond is another example, though less common. Its wide bandgap of 5.5 eV makes it a strong insulator at room temperature. But under extreme conditions, it can act as a semiconductor. Diamond is being studied for high-power and high-frequency uses because of its excellent thermal conductivity.
Here’s a simple comparison of these materials:
Property | Silicon | Germanium | Gallium Arsenide |
|---|---|---|---|
Bandgap (eV) | 1.1 | 0.66 | 1.43 |
Thermal Stability | High | Moderate | High |
Applications | Transistors, Solar Cells | Infrared Optics, Sensors | LEDs, Laser Diodes |
These examples show the variety of intrinsic semiconductors used in electronics. Each material has special benefits, making it good for specific uses.
Tip: When picking a semiconductor material, think about its bandgap, heat stability, and what you need it for.
Intrinsic vs. Extrinsic Semiconductors
Differences in Composition
The key difference between intrinsic and extrinsic semiconductors is their purity. Intrinsic semiconductors are pure materials with no added substances. They depend only on their natural ability to conduct electricity. Common examples include silicon and germanium.
Extrinsic semiconductors, however, are made by adding impurities to a pure material. This process, called doping, adds extra electrons or holes. For instance, adding phosphorus to silicon increases free electrons, creating an n-type semiconductor. Adding boron increases holes, forming a p-type semiconductor.
Type of Semiconductor | Composition | Example Materials |
|---|---|---|
Intrinsic | Pure, no impurities | Silicon, Germanium |
Extrinsic | Doped with impurities | Doped Silicon, GaAs |
This difference allows extrinsic semiconductors to be customized for specific uses.
Electrical Conductivity Comparison
Intrinsic and extrinsic semiconductors differ in how well they conduct electricity. Intrinsic semiconductors have equal electrons and holes, limiting their conductivity. Their ability to carry electricity depends on temperature. At room temperature, their conductivity is low.
Extrinsic semiconductors conduct electricity better because of doping. The added impurities increase charge carriers, like electrons or holes. For example:
The electrical resistance of doped silicon at room temperature is between 0.10 and 60 Ω-cm.
This resistance is much lower than that of insulators.
This higher conductivity makes extrinsic semiconductors better for electronic devices.
Note: Intrinsic semiconductors are great for learning basic physics, while extrinsic ones are better for practical uses.
Applications of Intrinsic and Extrinsic Semiconductors
Intrinsic semiconductors are mostly used in research. Their purity makes them ideal for studying basic semiconductor properties. They are also used in devices needing high precision.
Extrinsic semiconductors are widely used in electronics. Their adjustable properties make them essential for diodes, transistors, and circuits. For example:
N-type semiconductors improve solar cell efficiency.
P-type semiconductors are used in LEDs and rectifiers.
Application Area | Intrinsic Semiconductors | Extrinsic Semiconductors |
|---|---|---|
Research | Studying basic properties | Limited |
Electronics | Limited | Diodes, Transistors, Solar Cells |
Understanding these uses shows how intrinsic and extrinsic semiconductors work together to advance technology.
Practical Uses of Intrinsic Semiconductors
Applications in Electronics and Devices
Intrinsic semiconductors are very important in electronics. They conduct electricity only under specific conditions. This makes them perfect for precise devices like diodes, transistors, and sensors. These devices depend on the balance of electrons and holes in intrinsic semiconductors.
For instance, silicon is used in solar cells to turn sunlight into electricity. Its stable bandgap helps it convert energy efficiently. Germanium, another intrinsic semiconductor, is used in infrared detectors. These detectors capture light that humans cannot see, making them useful in cameras and optical tools.
Intrinsic semiconductors are also the base for advanced technologies. They are found in microchips used in computers and smartphones. Their purity ensures they work consistently, which is crucial for reliable devices.
Tip: When creating electronic devices, think about the material’s bandgap and how it reacts to heat for better performance.
Use in Research and Development
Scientists use intrinsic semiconductors to study basic properties. Their purity makes it easier to learn how semiconductors behave without interference. This helps researchers understand their behavior in different situations.
Many research projects focus on intrinsic semiconductors. These studies look at their thermal, electrical, and mechanical traits. Below is a table showing some key projects:
Project Title | Description |
|---|---|
Studies material properties in advanced packages. | |
Advanced Analytical Electron Tomography | Creates 3D methods to study devices at atomic levels. |
Multiscale Modeling and Validation | Builds models for semiconductor structures, focusing on their interfaces. |
Accurate Cure Kinetics and Mechanical Properties | Examines material traits for better packaging predictions. |
Nanocalorimetry Measurements | Studies tiny-scale heat behavior and process monitoring. |
Transport Property Measurements | Develops ways to measure heat and electricity movement in materials. |
These projects show how intrinsic semiconductors help improve technology. They provide knowledge about materials, leading to better device designs.
Challenges in Practical Applications
Using intrinsic semiconductors in technology has some difficulties. At room temperature, their conductivity is low. This limits their direct use in many devices. Often, they need to be modified or combined with extrinsic semiconductors for better performance.
Testing reliability is another issue. Devices made with intrinsic semiconductors must pass strict tests to ensure they work in real-life conditions. Accelerated Life Testing (ALT) is one method used. It stresses devices with heat, RF, and DC for long periods to check durability.
Here’s a table summarizing ALT details:
Key Component | Description |
|---|---|
Accelerated Life Testing (ALT) | Tests reliability using heat, RF, and DC stress. |
Testing Duration | Runs for hundreds or thousands of hours until failure. |
Statistical Significance | Tests many devices to gather accurate data. |
Extrapolation of Lifetime | Predicts how long devices will last based on failures. |
These challenges need careful planning and testing. Solving them can unlock the full potential of intrinsic semiconductors in today’s technology.
Importance of Intrinsic Semiconductors
Contribution to Modern Technology
Intrinsic semiconductors are very important for today’s technology. These pure materials are the base of many devices we use daily. Gadgets like smartphones, computers, and solar panels depend on them to work well. Their ability to control electricity makes them perfect for creating reliable parts.
For instance, silicon is a common intrinsic semiconductor. It powers the microchips in your devices, keeping them running smoothly. Germanium, another intrinsic semiconductor, is used in infrared sensors. These sensors are found in night vision cameras and thermal imaging tools. Without intrinsic semiconductors, these technologies wouldn’t exist.
They are also key in renewable energy. Solar cells, which turn sunlight into electricity, rely on intrinsic semiconductors. Their ability to capture and convert energy makes them essential for green technology.
Did You Know?
The semiconductor industry is worth billions, with intrinsic semiconductors at its heart.
Role in Understanding Semiconductor Physics
Intrinsic semiconductors help us learn about semiconductor science. Their purity lets scientists study electrons and holes without impurities getting in the way. This makes them great for research and teaching.
By studying intrinsic semiconductors, you learn about band structure, charge carriers, and heat effects. These ideas are the basics of semiconductor science. For example, understanding how electrons move between energy bands helps create better materials for electronics.
Researchers also use intrinsic semiconductors to test theories. They check models about electricity and temperature effects. This leads to new technology, like faster chips and better solar panels.
Learning about intrinsic semiconductors shows how devices work. This knowledge improves technology and sparks ideas for future inventions.
Knowing about intrinsic semiconductors helps you understand modern electronics. These pure materials have equal electrons and holes. This balance is important for devices like transistors and solar cells. They conduct electricity only in certain conditions, making them key for technology.
Intrinsic semiconductors are different from extrinsic ones because they are pure. Intrinsic semiconductors are great for research and precise tasks. Extrinsic semiconductors are better for everyday use because they conduct electricity better. Both types are crucial for advancing technology in many industries.
FAQ
What is the difference between intrinsic and extrinsic semiconductors?
Intrinsic semiconductors are pure materials without added impurities. Extrinsic semiconductors have impurities added to improve conductivity. Intrinsic ones are great for research, while extrinsic ones are used in devices like diodes and transistors.
Why is silicon commonly used as an intrinsic semiconductor?
Silicon has a bandgap of 1.1 eV, making it stable at room temperature. It is affordable, widely available, and forms strong bonds in its structure. These features make it ideal for electronics like solar panels and microchips.
How does temperature affect intrinsic semiconductors?
Heat gives electrons energy to move. This helps them jump from the valence band to the conduction band, increasing conductivity. At 0 Kelvin, intrinsic semiconductors act as insulators because electrons cannot move.
What are the main applications of intrinsic semiconductors?
Intrinsic semiconductors are used in research to study their properties. They are also found in devices like transistors, diodes, and solar cells. Their purity ensures they work accurately, making them useful for advanced technology.
Can intrinsic semiconductors be used in high-temperature environments?
Intrinsic semiconductors can handle moderate heat, but their conductivity rises with temperature. For very high heat, materials like diamond, with wider bandgaps, are better choices.
What are charge carriers in intrinsic semiconductors?
Charge carriers are electrons and holes. Electrons have a negative charge, while holes are positive spaces left when electrons move. In intrinsic semiconductors, these carriers are always equal, keeping the material neutral.
Why is germanium less common than silicon in modern electronics?
Germanium has a smaller bandgap (0.66 eV), making it less stable in heat. Silicon is cheaper, more available, and better for most electronics, so it is used more often than germanium.
How do intrinsic semiconductors contribute to renewable energy?
Intrinsic semiconductors like silicon are used in solar panels to turn sunlight into electricity. Their ability to capture and transfer energy makes them important for renewable energy systems.
Tip: When picking a semiconductor, think about its bandgap, heat stability, and purpose.
See Also
Exploring Integrated Circuits And Their Essential Parts
A Guide To Types And Categories Of Field-Effect Transistors
The Importance Of Force Sensitive Resistors In Technology
